In Vitro Diagnostic Regulation
A Quick Guide
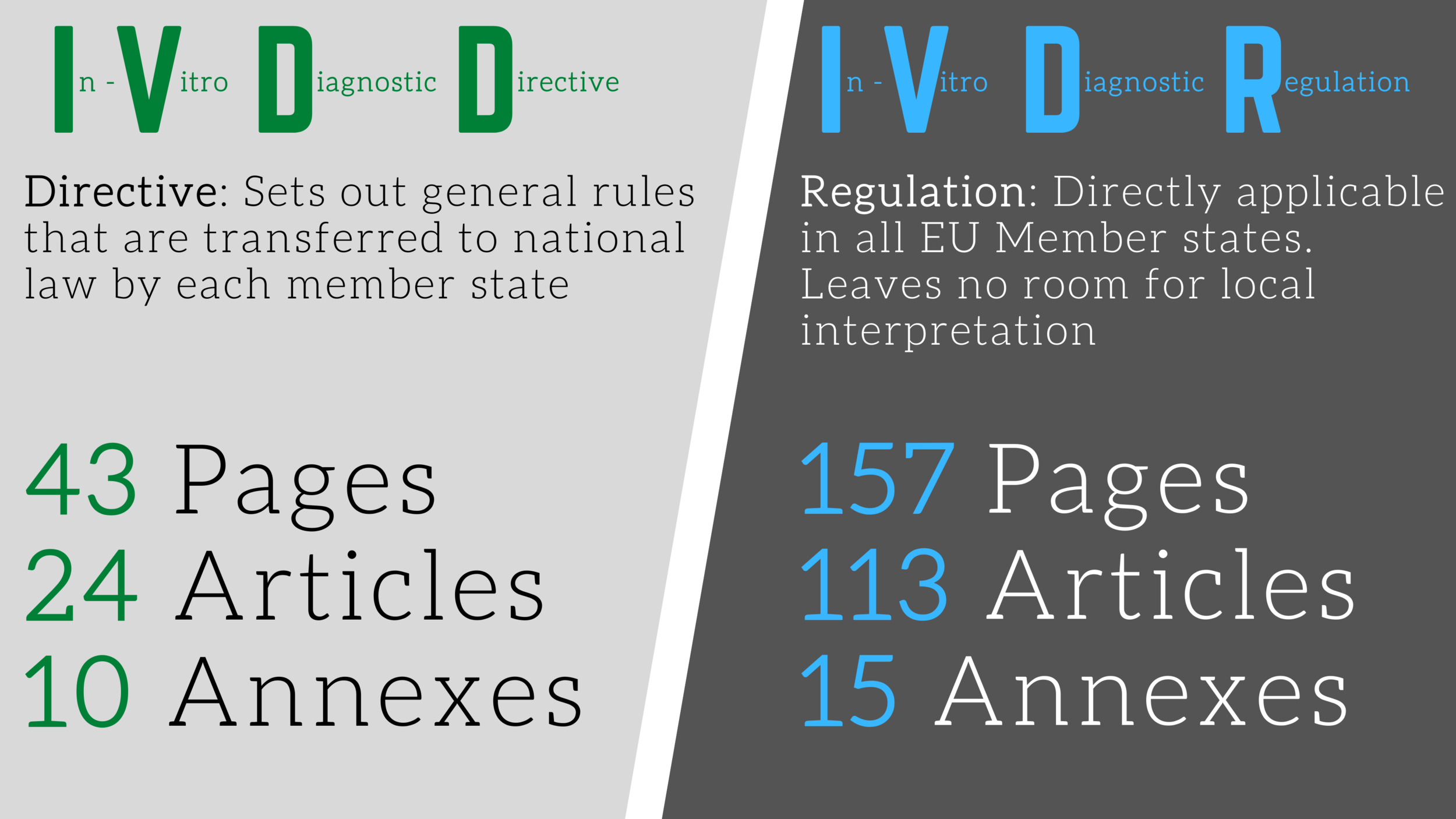
The European In Vitro Diagnostic Regulation (IVDR), which became applicable in May 2022, has significantly transformed the landscape for in-vitro diagnostic companies selling products within the EU. Companies that do not comply with the new requirements will no longer be able to place their products on the market or continue selling them once their transitional provisions expire.
Want to know the latest?
Sign up for our Regulatory Intelligence Newsletter!
The IVDR is a lot more complicated and comprehensive than its predecessor.
Considering both MDR and IVDR?
Compare them side by side, chapter by chapter, to determine how the requirements align.
We have created a comparison table to cut down on mistakes and confusion when comparing the regulations, providing an all-in-one view side-by-side.
Your Guide to the IVDR
This page provides an easy-to-follow guide on the implementation of the new In Vitro Diagnostic Regulation (IVDR 2017/746). It is our simplified overview, based on our extensive experience, and should be used only for guidance.
Your journey may be grouped into 5 Stages:
Device Classification
Economic Operators
Gap Analysis
Implementation
Verification & Final Check
Device Classification
First, it is important to confirm if the IVDR rules will impact your existing (or future) product classifications.
If you are looking for a detailed overview of the IVDR Classification Rules, we will soon release a tool to do just that. Please stay tuned.
Economic Operators
As part of the new MDR 2017/745 and IVDR 2017/746, Economic Operators (i.e. those involved in the importation and distribution of medical devices) are subject to new regulatory changes that affect their ability to conduct business within the European Economic Area (EEA). Economic operators may be Manufacturers, Importers, Distributors, or Authorized Representatives – each has unique requirements.
Although each Economic Operator is individually responsible for compliance with the new IVDR requirements, operators MUST CONFIRM that all previous operators in the distribution chain have also adhered to regulations. As a result of the new regulations, you may have several Economic Operator responsibilities.
The MDRG Economic Operators Checklist was created to enable you to understand and classify your company (and associated partners), and understand the implications.
Gap Analysis
Performing a thorough Gap Analysis is critical to minimize the amount of work you must do, focus on what is important, and assess your current level of compliance. A thorough, detailed gap analysis will generate a list of tasks for updating your procedures and quality documentation.
We are developing an IVDR Gap Analysis Tool that will support you in this process. Sign up to our newsletter or follow us on LinkedIn for updates.
Implementation
Implementing the changes you require for IVDR necessitates a structured approach. We have outlined the basic steps below.
Technical File
Your technical file must be updated per IVDR requirements. These can vary depending on the gap analysis you have previously done, and your classification.
Find more in: Annex II and Annex III
Post Market Surveillance (PMS)
With IVDR, monitoring the safety of your medical device after it has been released on the market has become increasingly important. You must review the new requirements, primarily defined in Chapter VII.
Find more in: Article 10
Use of UDI System
A requirement of the IVDR is that every medical device be assigned a Unique Device Identification – Device Identifier (UDI-DI) and a Unique Device Identification – Production Identifier (UDI-PI).
The purpose is to allow easy tractability across all medical devices. It will be one of the primary ways to identify products in the EUDAMED.
Post Market Performance Follow-up (PMPF)
A relatively new requirement, PMPF requires you to proactively collect and evaluate data on clinical performance, confirm its scientific validity, and ensure the continued suitability (and acceptability) of identified risks and the detection any new risks, based on the evidence collected.
This requires manufactures to carry out a PMPF as well as a clinical evaluation in accordance with Annex XII and Article 56.
Labelling
Labelling is a key component of the IVDR, considering the various languages and requirements that it will cover. Manufacturers are required to ensure their device is accompanied by information set out in Annex I (Section 20), in official EU language(s).
The language requirements are determined by the Member State in which the device is to be sold or made available to the user. There are key requirements, such that the label be easily legible, clearly comprehensible and the ink (or markings) be indelible.
Performance Evaluation Report (PER)
A key requirement of the IVDR, a PER is a systematic and planned process used to continuously generate, collect, analyse, and assess data pertaining to a device, in order to verify the safety and performance, including clinical benefits, when used as intended by the manufacturer.
Find more in: Annex XIII & MEDDEV 2.7.1
EUDAMED Registration
The EUropean DAtabase on MEdical DEvices (EUDAMED) is a secure, web-based databank that acts as a central source-of-truth for the exchange of information between various national competent authorities and the European Commission. Created in 2011, its purpose was to strengthen market surveillance and transparency of medical devices placed onto the EU market. Essentially, it is designed to provide a comprehensive but quickly accessible database of device information.
This includes:
Registration of manufacturers, Authorized Representative and devices
Declaration of conformity
Vigilance and traceability system
Labelling and instructions
Justification of the classification (Per Annex IX)
ISO Certificate / Proof of QMS
Clinical Investigations information
Additional documents (as required, dependent on the device)
The portal is not accessible to the public. At present, the EUDAMED is not online, however it is possible to access the latest Functional Specs. The European Commission has decided to move the release of the system until May 2022.
Risk Management (In accordance with ISO 14971)
There has long been an (unwritten) expectation that Manufacturers have a risk management system which conforms to EN ISO 14971. However, the In-Vitro Diagnostic Directive (IVDD) does not explicitly require this, nor does it contain an explicit requirement to employ risk management, other than for software devices.
The IVDR, however, contains an obligation (in Article 10 (2)), that Manufacturers establish, document, implement and maintain a system for risk management. Further details are listed in Annex I Chapter I (3). Although not explicitly requiring EN ISO 14971 in the IVDR, the requirements are very closely linked, to the point where EN ISO 14971 will become the minimum standard for device risk management.
Requirements include (but are not limited to):
risk management plan for each device
identification and analysis of hazards associated with each device
estimation of risk associated with the intended use and misuse
risk mitigation (reduction or elimination of risk)
assessment of production and post-market information on the risk assessment (i.e. Consider PMS/PMPF)
changes to control measures (e.g. safety by design, safety information) when required based on the above assessment
General Safety & Performance Requirements (GSPR)
Previously, in IVDD 98/79/EC, this was called the “Essential Requirements”.
The requirements are essentially a group of product characteristics, considered necessary by the EU authorities to ensure that any device will be safe and perform as intended throughout its life. Such requirements are common to other regulations globally, such as the Australian or Canadian essential principles.
To conform to Annex I of IVDR 2017/746, a GSPR checklist is a mandatory document and is one of the most fundamental pre-conditions to put any medical device on the EU market.
MDRG is currently creating an IVDR General Safety & Performance Requirements Checklist that contains a full table of the requirements, along with a list of Applicable Standards. Designed to be easy to use and follow, the template will save you many hours, headaches and potential mistakes.
Verification & Final Check
As the last step, it is important to ensure the changes made will meet the requirements of IVDR.
Internal audits will be necessary to do this as well as a final complete check (and recheck) to ensure you pass your actual audit.